Abstract
Background
VOD/SOS is an unpredictable, potentially life-threatening complication of conditioning regimens for HSCT or chemotherapy without HSCT. VOD/SOS with multi-organ dysfunction (MOD) may be associated with >80% mortality. One center with a primarily adult population (median age = 35.8 [range: 4-67] years), reported a Day +100 mortality rate of 79% in patients with unresolved severe VOD/SOS. For adult and pediatric patients, defibrotide is approved to treat hepatic VOD/SOS with renal or pulmonary dysfunction post-HSCT in the United States (US) and to treat severe hepatic VOD/SOS post-HSCT in the European Union. The European Society for Blood and Marrow Transplantation (EBMT) notes that clinical presentation of VOD/SOS differs between adult and pediatric patients, but most published results describe mixed age populations. Day +100 survival results specific to adults receiving defibrotide 25 mg/kg/day include those of a phase 2 dose-finding trial (33%, n=52) and a compassionate-use study (46.3%, n=141). Here, we report final adult subgroup outcomes data from the defibrotide expanded-access (T-IND) program, the largest prospective evaluation of defibrotide.
Methods
The T-IND was a multicenter, single-arm study designed to provide access to defibrotide prior to US approval. The original protocol required VOD/SOS diagnosis by Baltimore criteria or biopsy post-HSCT, with evidence of MOD (renal/pulmonary dysfunction). The study was amended to include patients without MOD (off-label), with VOD/SOS per modified Seattle criteria, or VOD/SOS following chemotherapy without HSCT (off-label). Defibrotide treatment (25 mg/kg/day) was recommended ≥21 days. Day +100 survival was analyzed for all adults, subgroups with and without MOD, and post hoc with VOD/SOS onset after Day +21 (ie, late-onset, per EBMT proposed criteria for adults). A post hoc exploratory analysis examined the relationship between Day +100 survival and time from VOD/SOS diagnosis to start of defibrotide treatment and included a trend test for treatment initiated on particular days post-diagnosis (Cochran-Armitage trend test).
Results
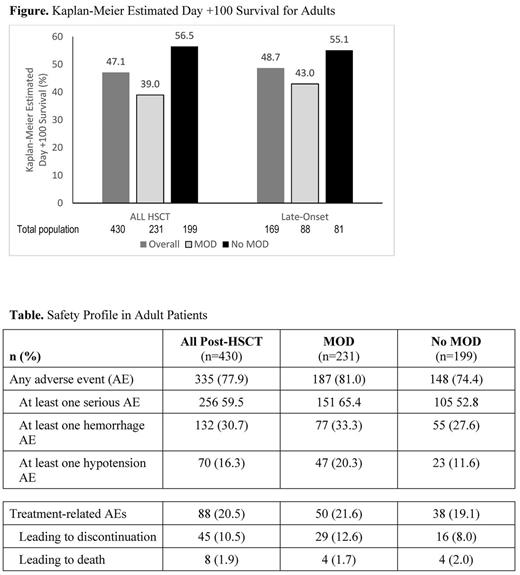
Of 1000 patients with VOD/SOS post-HSCT who enrolled in the T-IND study through April 2016 and received ≥1 dose of defibrotide, there were 430 adult patients: 231 had MOD and 199 did not. Median age at HSCT was 42.0 years (MOD subgroup, 41.0 years; no MOD subgroup, 43.0 years). Most common primary diseases were acute myelogenous leukemia (34.7%; MOD, 38.1%; no MOD, 30.7%) acute lymphocytic leukemia (22.1%; MOD, 20.8%; no MOD, 23.6%), non-Hodgkin lymphoma (8.4%; MOD, 6.5%; no MOD, 10.6%), and myelodysplastic syndrome (7.0%; MOD, 7.8%; no MOD, 6.0%). Most common graft-vs-host disease prophylaxes were tacrolimus (69.5%; MOD, 70.1%; no MOD, 68.8%), methotrexate (42.6%; MOD, 43.7%; no MOD, 41.2%), sirolimus (17.0%; MOD, 20.3%; no MOD, 13.1%), and cyclosporine (14.9%; MOD, 16.5%; no MOD, 13.1%). Characteristics were generally similar in the 169-patient late-onset subgroup (MOD, n=88; no MOD, n=81).
Kaplan-Meier estimated Day +100 survival was similar between all adults post-HSCT (47.1% [39.0% for patients with MOD]) and the late-onset adult subgroup (48.7% [43.0% for patients with MOD]; Figure). Exploratory post hoc analysis in the adult patient subset found that earlier defibrotide initiation post-VOD/SOS diagnosis was associated with higher Day +100 survival; however, the P value was not significant (P=0.055)
Of the 430 adult patients, adverse events (AEs) occurred in 77.9%, with treatment-related AEs in 20.5% (Table). Most common (>2%) treatment-related AEs (TRAEs) were gastrointestinal hemorrhage and epistaxis (3.5% each), pulmonary hemorrhage (2.3%), and hematuria and hypotension (2.1% each); most common (>1%) TRAEs leading to discontinuation were gastrointestinal hemorrhage (2.3%) and pulmonary hemorrhage (1.6%). No TRAEs led to death in ≥1%. In general, the safety profile was consistent for patients with late-onset VOD/SOS.
Conclusion
Final results from the T-IND present data from the largest adult VOD/SOS population (with or without MOD and with or without late-onset VOD/SOS) treated with defibrotide. The survival rate in adults with VOD/SOS post-HSCT is consistent with prior results for adults receiving defibrotide, and no new safety signals were observed.
Support: Jazz Pharmaceuticals.
Richardson: Oncopeptides AB: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Research Funding; Takeda: Consultancy, Research Funding. Antin: Gentium SpA/Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Ryan: Celator/Jazz: Employment, Equity Ownership. Liang: Jazz Pharmaceuticals, Inc.: Employment, Other: stock options. Hume: Jazz Pharmaceuticals, Inc.: Employment, Other: stock options. Tappe: Jazz Pharmaceuticals, Inc.: Employment, Other: stock options. Kernan: Gentium: Other: Received grants from Gentium during the conduct of the study and research was supported by The National Cancer Institute of the National Institutes of Health under award number P30 CA 008748, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal